.On the basis of reactivity of different metals with oxygen, water and acids as well as displacement reactions, the metals have been arranged in the decreasing order of their reactivities. This arrangement is known as activity series or reactivity series of metals. The basis of reactivity is the tendency of metals to lose electrons. If a metal can lose electrons easily to form positive ions, it will react readily with other substances. Therefore, it will be a reactive metal. On the other hand, if a meal loses electrons less rapidly to form a positive ion, it will react slowly with other substances. Therefore, such a metal will be less reactive.
(i) Which of the
following metals is less
reactive than hydrogen?
(a) Copper (b) Zinc (c) Magnesium (d)
Lead
(ii) Which of the following metals is
more reactive than hydrogen?
(a) Mercury (b) Platinum (c) Iron
(d) Gold
(iii) Which of the following metals reacts
vigorously with oxygen?
(a) Zinc (b) Magnesium (c) Sodium (d)
Copper

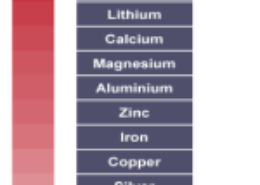
Answer ( 1 )
Answer.(a) Copper
Copper is placed below hydrogen in
activity series therefore, it is less
reactive than hydrogen.
Answer. (c) Iron
Iron is placed above hydrogen in
activity series, therefore it is more
reactive than hydrogen.
Answer (C) Sodium
Sodium metal react vigorously with
oxygen (O2 ) and water (H2O). A lot
of heat generates during the
reaction therefore sodium always
stored in kerosene