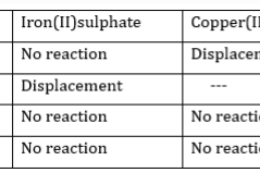
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows:
Question
Use the table given above to answer
the f0llowing questions about
metals A,B,C and D.
(a) Which is the most reactive metal?
(b) What would you observe if B is
added to a solution of Copper(II)
sulphate?
(c) Arrange the metals A, B,C and D
in order of decreasing reactivity.
in progress
0
Class 10 science
3 years
2021-12-19T18:04:12+00:00
2021-12-19T18:04:12+00:00 1 Answer
10 views
Enlightened 0

Answer ( 1 )
Answer.
(a) B is the most reactive metal
(b)If B is added to a solution of copper
(II) sulphate, displacement reaction
will take place. Blue colour of copper
sulphate will fade and red –brown
copper will settle down.
(c) The decreasing order of reactivity is:
B> A> C >D